Latest news from BBI LLC, pressure ulcer detection experts
The latest news from BBI LLC, the pioneers in biometric-sensor based medical devices…
September 2019
BBI LLC boosts its global team with appointment of Zoe Wood as Senior Clinical Manager
Zoe Wood has joined BBI LLC in the new position of Global Senior Clinical Manager. As BBI’s Global Senior Clinical Manager, Zoe’s primary role will be to develop and manage BBI’s clinical and health economics research, post market studies and publication plan. She will also support the marketing team in developing evidence-based claims and messaging for BBI’s SEM Scanner, and the global sales organisations in presenting clinical and HE data to expert groups, customers and other commercial organisations.
Kate Hancock, Global Vice President of Marketing and Communications at BBI said: “A Senior Clinical Manager is essential for BBI’s increased growth and focus in furthering our clinical and health economic data. Zoe brings a great deal of experience and is a welcome and important addition to our team.”
Zoe joins BBI LLC with more than 20 years’ experience in clinical care, having a Clinical Nursing Master’s Degree - Wound Healing and Tissue Repair and holding positions at high profile organisations including Healogics, Archimed and BUPA. Commenting on her appointment, she said: “I have seen first-hand the impact of pressure ulcers on two members of my family, yet these could so easily have been prevented. BBI’s technology has the potential to help all healthcare professionals revisit their current pressure ulcer prevention strategies, and I am very excited to join this innovative company.”

September 2019
BBI LLC extends its partnership with RCSI on diabetic foot ulcer prevention research
Manchester, UK, and Dublin, IRELAND, - BBI LLC (Bruin Biometrics), a developer of innovative sensor-based diagnostic products, and the Skin Wounds, and Trauma (SWaT) Research Centre at RCSI (Royal College of Surgeons in Ireland), announced today that they have agreed to extend their partnership to undertake a number of collaborative projects that will employ BBI’s proprietary biocapacitance technology, the SEM Scanner.
The research programme will continue to explore biocapacitance science as a novel methodology supporting Diabetic Foot Ulcer (DFU) prevention. It seeks to radically alter the progression of the disease while contributing to the biological understanding of DFU development, the underlying pathophysiology and biomarkers, such as sub-epidermal moisture (SEM). The ultimate aim is to prevent Diabetic Foot Ulceration and reduce the prevalence of this significant public health problem.
The planned study includes the use of BBI’s SEM Scanner to identify Diabetic Foot Ulceration before it is visible. This clincial research will enable the translation of evidence into contempoaray clinical decision-making, and those at risk of developing DFUs will ultimately benefit from this approach, improving thier quality of life, and saving services money.
Highly significant clinical data has been found in the area of pressure ulcer prevention
“The team at the Skin Wounds and Trauma (SWaT) Research Centre at RCSI is delighted with the opportunity to extend our partnership with BBI LLC. Our research collaboration to date has yielded highly significant clinical data mainly in the area of pressure ulcer prevention. The planned study will extend our research relationship into the area of Diabetic Foot Ulceration and how it can be prevented through earlier identification by the analysis of the role of biocapacitance technology in DFU prevention. At the heart of our research are patient outcomes that will improve patient quality of life. Our aim is that this study will continue to make a significant mark on the area of earlier identification of Diabetic Foot Ulceration with the resultant outcome that the lives of patients and their families are improved ’ said Professor Zena Moore, Director, SWaT Research Centre. RCSI.
“I am delighted to extend the partnership with the RCSI and I look forward to the new advances our continued collaboration will bring in the prevention of this pernicious clinical problem,” said Martin Burns, CEO, BBI LLC
Diabetes is a growing epidemic, with associated complexity of treatment and management from a wound care perspective, prevalence of foot ulceration reaches as high as 10%. Diabetes is the leading cause of non-traumatic limb amputation in the world. Within 18 months following amputation, almost 50 percent of individuals will develop ulceration on the other limb and of these, 58% will have further amputations within three to five years. It’s worthy of note that the three-year mortality rate after the first amputation is between 20 and 50 percent. This is down to the motor and sensory neuropathy associated with diabetes, resulting in damage to the foots nerve supply. This chain of events leads to further bone, joint and soft tissue damage, which can lead to irreversible cell destruction.
Quality of life is threatened through mobility loss, subsequent social functioning and intractable pain experienced in almost 50% of these patients. Within healthcare, diabetic foot ulceration represents estimated costs of €4–6 billion in light of a 1-1.4 million prevalence, placing a significant burden on resource.
The SEM Scanner received European CE Mark approval in 2014 and Health Canada clearance in 2016 and is in full commercial use in Australia, New Zealand, Canada and the European Union including the UK, Belgium and Spain with additional markets expected to be opened in 2019. Additionally, FDA Authorisation to market the SEM Scanner in the USA was received in December 2018.
December 2018
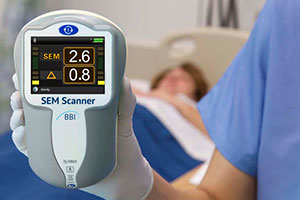
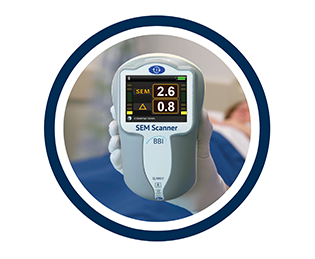
SEM Scanner detects pressure sores before they're visible
Care and Nursing Essentials editor Victoria Galligan spoke to the team at Bruin Biometrics, who are behind the SEM Scanner. The scanner can detect pressure damage before it is visible to the naked eye…
Could you outline the workings of the SEM Scanner - how does it detect the moisture under the skin?
The SEM Scanner is a, hand-held portable device that has been designed to measure sub-epidermal moisture (SEM) (also known as localised oedema) which is an invisible precursor to the development of incipient pressure damage.
The analogy we like to use is one of an oil tanker moving through water. The oil tanker is the actual damage to the skin and tissue, but the big wave in front of the oil tanker is the leading indicator of damage. The Scanner therefore gives a leading warning of skin and tissue being in trouble even before you are able to see that trouble developing on the skin’s surface.
The SEM Scanner measures to a depth of approximately 4mm and through a series of readings on the sacrum or heel calculates a Delta value indicating the health of the tissues.
A SEM delta value of <0.5 is indicative of low site-specific SEM variance, suggesting the tissue is healthy, values >0.5 reflects increased site-specific SEM variance suggesting developing pressure damage.
How early can it detect ulcers and how does this affect treatment and healing times?
The SEM Scanner has been found, when used as an adjunct to current standards of care, to detect tissue damage on the sacrum and heels five days (median)(1) before it becomes visible on the skin surface. Assessment takes from one minute to under 10 minutes (approximately) and so is easily incorporated into existing clinical workflows.
The SEM scanner has been proven to result in an 86.2% reduction in Hospital Acquired Pressure Ulcers(2). As one pressure ulcer can lead to an additional five to eight in-patient days3, use of the SEM Scanner therefore not only helps to keep patients free from harm, but also frees up NHS beds, reduces nursing time to treat patients with PUs, and saves Care Facilities money.
Which healthcare providers will use the SEM Scanner – in a hospital, GP surgery, in nursing homes etc?
All of the above! To date in the UK alone, more than 20 different healthcare facilities have experience of using BBI’s SEM Scanner within a number of different settings including:
- Medical
- Orthopedic
- Elderly Care
- Intensive Care
- Vascular Care
- End of Life Care
- Surgical
- Stroke / Rehabilitation
Could you outline a case study or trial which has been successful?
Virgin Care, which provides services to the NHS, experienced a 95% drop in the pressure ulcer rate during a service redesign evaluation of the SEM Scanner at Farnham Community Hospital in Surrey. (4)
Marie Curie, Newcastle, the first hospice in the UK to gain experience with the SEM Scanner, revealed a 47% reduction in hospice acquired pressure ulcer (HAPU) incidence rate in the first six months of use (November 2017 – April 2018). Use of the SEM Scanner enabled the team to react earlier than when had they relied purely on the Waterlow Risk Assessment and visual skin inspections. (5)
Does the SEM Scanner require training, or can most medical professionals use it?
The SEM Scanner can be used by most, if not all medical professionals and health care assistants. It has been designed by a clinician for clinicians and as such has received high ratings for ease of use and implementation into workflow to promote coordination of care.
This was substantiated in the Marie Curie experience, where 92% of the staff that participated (which included including staff nurses, healthcare assistants and Ward Sisters) said the SEM Scanner is easy to learn(5).
What is the cost of the SEM Scanner and how do people place an order?
The SEM Scanner costs £5,835 to purchase. Rental options are also available. Use of the Scanner delivers cost savings within the first year of use.
To place an order, contact the BBI sales team on info@bruinbiometrics.com or call 01625 238895.
1. Okonkwo H. et al. (2018) Evaluation of a novel device using capacitance of the detection of early pressure ulcers (PU), a multi-site longitudinal study. Accepted and presented at NPUAP 2018

